Home » Lab Capabilities » Dissolution Methods » Dissolution Theory

Pharmaceutical Dissolution Testing Theory
Dissolution is the process of a dispersion / dissociation of a solute in a solvent, forming a chemically and physically homogeneous dispersion at the molecular level called a solution. This process is controlled by the affinity between the solid and the liquid.
Testing dissolution of pharmaceutical dosage forms as defined by US and European pharmacopoeia, is an important in-vitro test to provide critical in-vitro drug release information. The obtained results are used for both quality control purposes, i.e., to assess batch-to-batch consistency of both oral and non-oral dosage forms, and drug development, i.e., to predict in-vivo drug release profiles and support the development of new formulations.
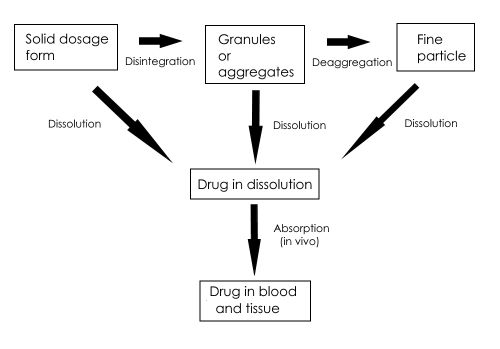
When a pharmaceutical dosage form is administered, the rate at which it releases the drug, or active ingredient, is critical to ensure that the drug is delivered properly. Solid dosage forms undergo dissolution in biological media followed by absorption of the medicine into the bio-circulation. The dissolution of a tablet can be schematically represented as follows:

Avivia is an independent pharmaceutical development contracting organization in the Netherlands offering a unique, complementary range of pharmaceutical services in the areas of:

The rate at which the solid drug is released is called the dissolution rate. Almost all drug forms have a dissolution rate; tablets, capsules, powders, creams, skin patches, implants, depot injections and others, all release their drugs so they can be taken up by the body.
In the figure above, the dissolution rate of, for example, a drug in tablet form can be the rate determining step before the drug enters the blood. However, if the drug enters the digestive tract as a solid, two speed factors are possible: the solid must first dissolve and then cross the gastrointestinal membrane.
Highly water-soluble substances make the diffusion and / or the active transport through the membrane the speed-determining step. For poorly soluble substances, the dissolution rate or the disintegration of the undissolved portion is the speed determining step. In intermediate cases, both factors determine the rate of drug absorption into the blood.
If dissolution is the speed determining step, it will be clear that with solutions (syrup, elixir) the drug can be absorbed into the blood most quickly. A drug given in solid form will take the longest to get into the blood. The sequence of dissolution rate, and thus absorption into blood, for different administration modes from fasted to slowest: solution > suspension > capsule > tablet > coated tablet.
Dissolution rate is a kinetic process. A solute can have poor solubility in a solvent, but the dissolution rate can be high. Conversely, a solute can be very soluble, but still take a long time to reach the final saturation concentration.
The dissolution rate can be expressed by the Noyes–Whitney equation:
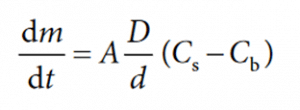
Where:
dm/dt = solute dissolution rate (kg.s-1)
m = mass of dissolved material (kg)
t = time (s)
A = surface area of the solute particle (m2)
D = diffusion coefficient (m.s-1)
d = thickness of the concentration gradient (m)
Cs = particle surface (saturation) concentration (kg or moles/L)
Cb = concentration in the bulk solvent/solution (kg or moles/L).
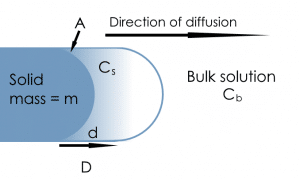
Dissolution, at a rate dm/dt, takes place from a solid with mass = m and surface area = A, from the saturation concentration at the particle surface (Cs) to the concentration in the bulk solution (Cb). Concentration follows a gradient d with a coefficient D.
Where:
dm/dt = solute dissolution rate (kg.s-1)
m = mass of dissolved material (kg)
t = time (s)
A = surface area of the solute particle (m2)
D = diffusion coefficient (m.s-1)
d = thickness of the concentration gradient (m)
Cs = particle surface (saturation) concentration (kg or moles/L)
Cb = concentration in the bulk solvent/solution (kg or moles/L).
Dissolution, at a rate dm/dt, takes place from a solid with mass = m and surface area = A, from the saturation concentration at the particle surface (Cs) to the concentration in the bulk solution (Cb). Concentration follows a gradient d with a coefficient D.
The Noyes-Whitney equation provides much practical information relevant to the solution process. If we look at the equation parameters, we see that the equation predicts the following:
Surface area (A) increases as particle size decreases: the dissolution rate (dm/dt) will be faster with smaller particles. Micronization particles of the active ingredient particles will usually speed up dissolution.
Decreasing the diffusion gradient (d) by removing dissolved molecules from the particle surface more quickly by increasing the stirring speed during the dissolution test, will increase dissolution rate dm/dt.
The diffusion coefficient, D, is inversely proportional to viscosity; D and thus dissolution rate dm/dt, will decrease with increasing solvent viscosity.
Altering the solvent pH can affect the surface/saturation concentration, Cs if the active ingredient is ionizable. Depending on the characteristics of the solvent and the active ingredient, this change could either increase or decrease Cs, and with that change the concentration gradient and the dissolution rate.

Sink Conditions
If Cb is small, Cb < 0.15 Cs, then D/d is proportional to Cs ((Cs – Cb) «Cs). The Noyes Whitney equation may then be simplified to:
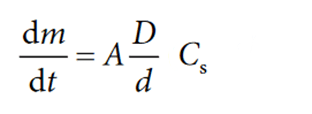
This simplified formula is mostly used as the equation for “sink conditions”, which implies that “sink” conditions exist during the dissolution process.


