Home » Lab Capabilities » Pharmaceutical Development » Dosage Forms

Pharmaceutical Dosage Forms
Pharmaceutical dosage forms play a pivotal role in the effective delivery of medicinal agents, ensuring their safe and efficient administration to patients. These forms serve as the carriers or vehicles for pharmaceutical substances, transforming raw drug materials into organized and standardized products that can be easily and conveniently consumed. The diversity of dosage forms reflects the dynamic intersection of pharmaceutical science, technology, and patient-centric healthcare.
In the realm of pharmaceuticals, dosage forms encompass a wide array of preparations, each tailored to meet specific therapeutic needs and patient preferences. From traditional tablets and capsules to innovative transdermal patches and sustained-release formulations, the design and development of dosage forms require a nuanced understanding of drug properties, patient characteristics, and regulatory considerations.
This multifaceted field involves the integration of various disciplines, including pharmaceutics, pharmacokinetics, and formulation science, to optimize the delivery of pharmaceutical agents. The goal is to achieve maximum therapeutic efficacy with minimal adverse effects, ensuring patient adherence to prescribed regimens. Moreover, dosage forms play a critical role in enhancing drug stability, bioavailability, and overall patient experience.
Tablet
A tablet is a compressed solid pharmaceutical dosage form often destined for oral delivery, but tablets for other routes of administration (e.g. vaginal) also exist. They contain the drug substance to be applied mixed with excipients including diluents or bulking agents, binding agents, flavours, sweeteners, pigments, desintegrants, lubricants and glidants. Tablets can be coated which has several advantages, including easier administration by the patient, an added functionality of controlled release of drug substance, and improving physicochemical stability and thereby shelf life of the product. For patient compliance and manufacturability, most tablets are round or oval-shaped. Using tablets to deliver drug to patients can be preferable to using liquid formulations, as physicochemical and microbiological stability are often better ensured, and fixed unit doses can be administered to the patient.
Amongst others, the following tablet types are available based on release profile:
- Orodispersible tablets
- Chewable tablets
- Immediate release tablets
- Gastro-resistant/Enteric coated tablets
- Extended release tablets

Orodispersible tablets are designed to fall apart within the mouth and thus must disintegrate very rapidly. Chewable tablets must do the same, but with the help of chewing. Immediate release tablets can be considered the regular tablets that will have to be swallowed. Drug substance release rate should not be appreciably affected by the composition of the tablets. Gastro-resistant or enteric coated tablets are designed to release the drug substance after the tablet has passed the gastric section of the gastrointestinal tract, as a means of preventing stomach acid degradation of the drug substance or gastric side effects. Extended release tablets release drug substance over an extended amount of time and are often used to improve patient compliance.
Capsule
A capsule is a pharmaceutical dosage unit consisting of either an outer “hard” capsule shell containing either loose or prepacked solid drug substance and excipient particles, or a soft capsule with a closed outer surface and a liquid filling. Like tablets, they are primarily used to deliver drug orally. The outer capsule shell is made of a gelatinous base, often gelatin or a starch derivative with or without softening agents. As with tablets, common excipients are diluents or bulking agents, glidants, pigments, and, in the case of liquid filled soft capsules, preservatives. Capsule shells can also be film coated in order to ease patient administration, control release of drug substance and to improve physicochemical stability and thereby shelf life of the product.
Amongst others, the following capsule types are available based on release profile:
- Immediate release capsules
- Gastro-resistant/Enteric coated capsules
- Extended release capsules

Immediate release capsules can be considered the regular capsules that will have to be swallowed. Drug substance release rate should not be appreciably affected by the composition of the tablets. Gastro-resistant or enteric coated capsules are designed to release the drug substance after the capsule has passed the gastric section of the gastrointestinal tract, as a means of preventing stomach acid degradation of the drug substance or gastric side effects. Extended release capsules release drug substance over an extended amount of time and are often used to improve patient compliance.
Oral solution
When oral delivery is desired but solid formulations are not acceptable due to swallowing difficulties, an oral solution may be a better option for patient compliance and convenience. The simplest and therefore most desirable form of an oral liquid is an oral solution, a single-phase system into which the drug substance and accompanying excipients are dissolved into a vehicle, most often purified water. Eligible excipients for oral solutions include the vehicle, pH regulators or buffers, microbiological and chemical preservatives, taste maskers/modifiers, thickeners, and pigments. By definition all compounds must be in solution, which can be challenging for certain drug substances. Often, solubility is increased by adjusting the pH (in the case of an ionizable drug substance), adding cosolvents or complexing agents to the vehicle or adjusting the vehicle entirely (i.e. an oleaginous vehicle). As the sense of taste of a drug substance is increased when it is in solution, taste masking is even more important in oral solutions than in solid oral dosage forms. Solutions may be formulated in the form of syrups containing a high amount of sugar, or sweet-tasting polyols may be used instead. Viscosity also affects taste and mouthfeel, so thickeners can be used to aid in improving organoleptic properties of the formulation as well. As drug substances in solution are generally more prone to chemical degradation, controlling rate of degradation by adding in preservative compounds is of increased importance in oral solutions.
Oral suspension
When a drug substance is to be delivered in an oral liquid formulation but has a prohibitively low solubility, or hydrolyzes rapidly when in aqueous solution, it may instead be formulated into an oral suspension. A suspension describes a mixture of a vehicular liquid with solid particles containing the drug substance, amongst others. An important downside of oral suspensions is that it requires proper handling by the patient upon administration, being shaken for use as to ensure proper dosing from start to end. However, compared with oral solutions, oral suspensions often show better chemical stability of the drug substance and fewer problems with taste masking, as the drug substance is (mostly) in solid state within the vehicle. Eligible excipients for oral suspensions include the vehicle, pH regulators or buffers, microbiological and physicochemical preservatives, taste maskers/modifiers, thickeners, deflocculants, and pigments. The role of thickeners is amplified for oral suspensions as they increase vehicle viscosity, affecting sedimentation rate and the ability to redisperse the oral suspension. Deflocculants or peptizers are added for the purpose of aiding redispersion of sediment. As with oral solutions, pH buffers and preservatives are of importance for physicochemical and microbiological stability. pH may be selected in such a way as to make the drug substance as insoluble as possible within the vehicle.

Oral emulsion
When a drug substance has low chemical stability or solubility in aqueous medium, an oral emulsion may instead be used. An emulsion describes a mixture of two or more immiscible liquids. Often, oral emulsions are presented in the form of oil-in-water emulsions and drug substance may be present in both phases of the mixture. Like with suspensions, an important downside of oral emulsions is that it requires proper handling by the patient upon administration, being shaken for use as to ensure proper dosing from start to end. Eligible excipients for oral suspensions include the vehicle (both phases), emulsifiers, pH regulators or buffers, microbiological and physicochemical preservatives, taste maskers/modifiers, thickeners, and pigments. Emulsifiers are of paramount importance for the physical stability of emulsion systems.

Injection
Injections are sterile liquid dosage forms administered directly into the body via a needle and syringe. This route allows for rapid drug action and is used when oral administration is not feasible. There are several types of injections, including intravenous (IV), intramuscular (IM), and subcutaneous (SC). The formulation of injections can include solvents, stabilizers, preservatives, and buffers to ensure stability and compatibility with the body’s tissues. Injections must be prepared under strict aseptic conditions to prevent contamination and infection.
Gel
Gels are semi-solid dosage forms that contain the drug dispersed within a gelatinous base. They are used for topical application to the skin or mucous membranes and provide localized drug delivery. Gels are formulated to be easily spreadable and can offer cooling and soothing effects. Common excipients in gels include gelling agents, preservatives, and stabilizers. Gels can be used for a variety of conditions, including inflammation, pain relief, and skin conditions

Cream
Creams are semi-solid emulsions that contain the drug dissolved or dispersed in a water-in-oil or oil-in-water emulsion base. They are used for topical application and provide a balance between an oily ointment and a water-based gel. Creams are typically used to treat skin conditions, such as eczema, rashes, and fungal infections. Excipients in creams can include emulsifiers, preservatives, humectants, and stabilizers. The choice of base affects the absorption rate and the ease of application.

Ointment
Ointments are semi-solid preparations that are primarily composed of oils and fats, with the drug dispersed throughout the base. They provide a protective barrier on the skin and are used to deliver medication to a localized area. Ointments are often used for dry, scaly skin conditions, as they provide a moisturizing effect. Excipients in ointments can include hydrocarbons, waxes, and emulsifying agents. They are designed to stay on the skin longer than creams, providing prolonged drug action.

Paste
Pastes are semi-solid dosage forms that contain a higher proportion of solid material than ointments, resulting in a thicker consistency. They are used for protective and therapeutic purposes on the skin, forming a barrier against moisture and irritants. Pastes are commonly used for diaper rash and other skin conditions where a protective layer is beneficial. Excipients in pastes include powders, oils, and waxes. Their thick consistency allows them to stay in place on the skin for extended periods.

Eye Drop
Eye drops are sterile liquid preparations intended for instillation into the eyes. They are used to treat various ocular conditions such as infections, allergies, glaucoma, and dry eyes. Eye drops must be isotonic with the natural fluids of the eye and have a pH that is compatible with the eye to avoid irritation. Excipients in eye drops can include preservatives, buffers, and viscosity-enhancing agents. They must be formulated to be sterile and free from particulates to prevent ocular infections.

Eye Ointment
Eye ointments are sterile, semi-solid preparations intended for application to the conjunctiva or eyelids. They provide a prolonged contact time of the drug with the eye compared to eye drops. Eye ointments are used to treat infections, inflammation, and dry eye conditions. Excipients in eye ointments can include petrolatum, lanolin, and mineral oil. They must be sterile and formulated to minimize irritation while providing effective drug delivery to the eye.
Nasal Drop/Spray
Nasal drops and sprays are liquid preparations administered into the nasal cavity. They are used for local treatment of conditions such as congestion, allergies, and infections, as well as for systemic absorption of certain medications. Nasal drops and sprays provide rapid drug absorption through the nasal mucosa. Excipients in nasal formulations can include preservatives, buffers, and solubilizers. They must be formulated to be non-irritating and to deliver an appropriate dose with each administration.
Ear Drop
Ear drops are liquid preparations intended for instillation into the ear canal. They are used to treat local conditions such as ear infections, inflammation, and earwax buildup. Ear drops must be formulated to be non-irritating and isotonic with the natural fluids of the ear. Excipients in ear drops can include solvents, preservatives, and viscosity-enhancing agents. They are designed to provide effective drug delivery while minimizing discomfort.

Suppository
Suppositories are solid dosage forms intended for insertion into the rectum, where they dissolve or melt to release the medication. They are used for local treatment of conditions such as hemorrhoids or for systemic absorption when oral administration is not feasible. Suppositories can provide a relatively quick onset of action and are useful for patients who cannot tolerate oral medications. Excipients in suppositories include bases such as cocoa butter, polyethylene glycols, and glycerinated gelatin. They are designed to melt at body temperature and provide effective drug release.
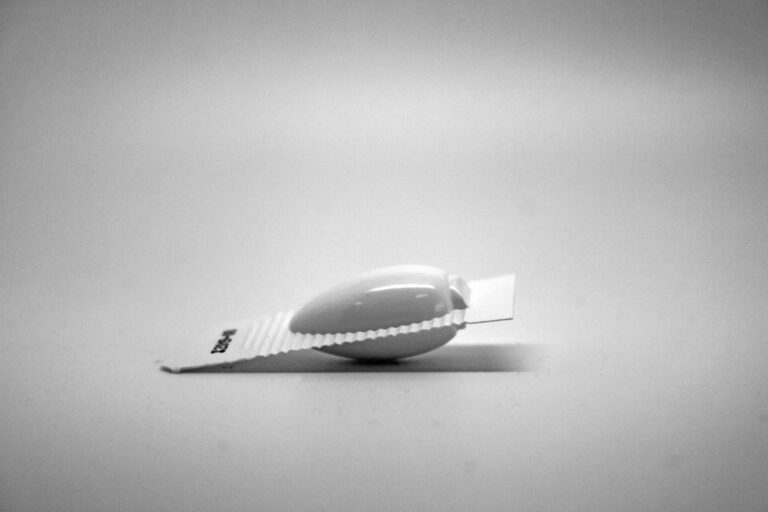
Ovule
Ovules are solid dosage forms intended for insertion into the vagina, where they dissolve or melt to release the medication. They are used to treat local conditions such as infections and hormonal imbalances. Ovules provide targeted treatment with minimal systemic absorption, reducing the risk of side effects. Excipients in ovules can include bases such as glycerinated gelatin and polyethylene glycols. They are formulated to provide effective drug delivery and patient comfort.
Pessary
Pessaries are solid dosage forms similar to ovules but are used to provide mechanical support to the vaginal walls or to deliver medication locally. They are used to treat conditions such as vaginal prolapse or infections. Excipients in pessaries include bases that dissolve or melt at body temperature, providing effective drug release or mechanical support. They are designed for ease of insertion and patient comfort.
Inhalation Device
Inhalation devices are used to deliver medication directly to the lungs via the respiratory system. These devices include metered-dose inhalers (MDIs), dry powder inhalers (DPIs), and nebulizers. Inhalation devices provide rapid onset of action and high local drug concentrations, making them ideal for respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD). Excipients in inhalation formulations can include propellants, stabilizers, and dispersing agents. The devices are designed to deliver a precise dose of medication with each use, ensuring effective treatment.
Avivia offers solutions for formulation challenges, either in the form of on-off problem-solving or in the form of a full pharmaceutical formulation development procedure. Interested? Get in touch with us!


